Competitors
Boston Kpro
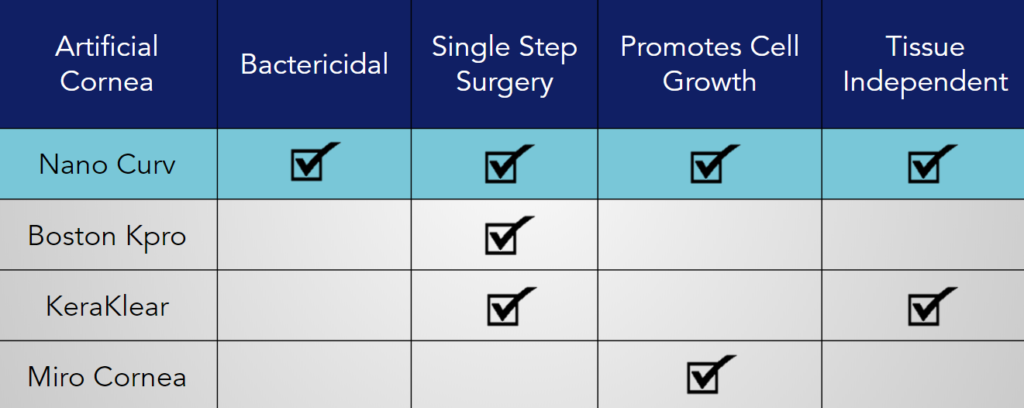
The Boston Kpro Type I is the current leader in the artificial cornea market in the United States. The device is composed of three parts: a front plate, back plate, and titanium locking c-ring. Its design allows the artificial cornea to be transplanted under a single step surgical procedure. During the transplantation, a donor corneal tissue is required in order to suture the device to the eye. Compared to other devices currently available on the market, Boston Kpro has shown its effectiveness in visual acuity retention rates after a long period of time.
KeraKlear
Another competing artificial cornea is the KeraKlear Kpro. The device has a novel foldable design and can be transplanted in a non-penetrating, single step surgery. Because it is non-penetrating, it does not require a donor tissue. While the non-penetrating design has its benefits, it also has drawbacks. The KeraKlear cannot heal more serious cases of corneal blindness, such as when chronic inflammation is present in the eye.
Miro Cornea
A newer keratoprosthesis, the Miro Cornea, is a single piece device composed of a soft hydrophobic acrylic material. The artificial cornea has a thin circular haptic with an optic cylindrical part that penetrates through the center. Similar to the Boston Kpro, the Miro Cornea is limited by the need for donor tissues in order to suture the device.To promote integration between the device and donor tissues, the haptic is coated with a material that helps with cell adhesion. Furthermore, the haptic is designed with holes that support diffusion of nutrients between the donor tissue and patient’s eye.
Why choose?

Compared to the Boston Kpro and Miro Cornea, our device overcomes one major limitation — the requirement for donor tissues for transplantation. Without such a limitation, our artificial cornea is more accessible and can reach a wider global market. While the KeraKlear also does not require donor tissues, they are hindered by their foldable design. They can be transplanted through a non-penetrating surgery, but they cannot be used to treat cases where there is swelling or inflammation in the eye. Furthermore, our artificial cornea has functionalized surfaces due to our patented nano-technology. The functionalized surfaces grant added benefits that are not found in our competitor’s devices.
